Studytrax and REDCap are the only two electronic data capture (EDC) systems designed for academic research and disease organizations, rather than the pharmaceutical industry. Despite the shared audience, Studytrax and REDCap have distinctly different development aims and feature sets.

Three Steps To Choose What Fits Best For You.

Which Requirements Align Best?
Best Fit For...
• Participants:
Participants enter data, often within a longitudinal study design. They benefit from incentives, regular communication, education and information about the study, and may enroll in other studies when available.
• Data Utilization
Ease of data analysis plays key role, and data may be used for instant reporting, day-to-day operations, decision-making, and/or providing information or education to participants.
• Study Design:
Design may include a mix of planned (e.g., baseline, months 1, 2, and 3) and unplanned (e.g., adverse events, diaries) events, may include custom follow-up or triggered events.
• Data Definition:
Data capture may involve interdependent data relationships, and logic-driven validation is required. Repeating forms have a longitudinal nature and require different types of validation to ensure accurate capture (learn more here), such as tumors, seizures, diagnoses, medication use, diaries, etc.
• Reporting:
Automated reporting helpful to minimize administrative demands (e.g., progress, IRB, AE, safety reports) and ongoing evaluation of data (e.g., table of main outcomes).

Best Fit For...
• Participants:
Participants either do not enter data or, if they do, the study design is cross-sectional. There’s no need to pay, incentivize, communicate, educate, or enroll them in additional studies. Participants are highly motivated, and the data entry burden is minimal.
• Data Utilization
Data is aggregated annually or biennially for analysis, with no urgency for disseminating results, and it isn’t used for day-to-day operations, decision-making or informing participants.
• Study Design:
Design is primarily planned events (e.g., baseline, months 1, 2, and 3), with few, if any, custom follow-up events (e.g., adverse events, diaries) or nested triggered events.
• Data Definition:
Straight-forward data capture, manual validation is acceptable, and either no repeating forms or if forms repeat all data is associated with a single visit (e.g., "enter your 5 favorite foods", as opposed to longitudinal information such as tumors, seizures, diagnoses, medication use, diaries, etc.).
• Reporting:
Minimal reporting needed and manual, delayed process sufficient as typically just occasional IRB report on basic demographic data such as age, sex, race and other associated participant characteristics.

What's More Like Me? Questions To Ask.


Tend to have at least one active study, new studies can run concurrently, research plays an important role in career goals, and publications / grants serve as a measure of productivity.
Tend to run one study every 2 to 5 years, each study runs sequentially, with research forming a smaller part of career goals, and publications / grants serve as a less critical measure of productivity.

-
Does the data entry experience motivate participants to stay enrolled?
-
Are staff bogged down by frustrating, repetitive, or error-prone data entry?
-
Does administrative work—like reports and documentation—drain staff time?
-
How simple is it to extract and analyze data, and build manuscript tables?
-
What's the cost of siloed data, participants, and event calendars across studies?
Questions About Whether REDCap Meeting Your Needs.
What Are The Benefits?


Save Time
-
Work More Effectively
- Automate administrative tasks (e.g., participant payments, notifying participants, event/invoice documentation, etc.)
- Directly connect any number of studies and patient registries
-
Eliminate Redundant Work
-
Work in a unified environment where all design elements, analyses, manuscripts, etc. can be copied or reused.
-
Eliminate duplicate data entry across studies
-
Engage Participants
-
Minimize Dropouts And Incomplete Data
- Incentivize active participation, provide immediate feedback
- Securely communicate with participants and between staff
-
Personalize Participant Experience
-
Deliver to participants disease and study information based on their own data with dynamic updates.
-
Allow participants to select their preferred rewards
-


Leverage Data
-
Eliminate Reporting Headaches
- Automate IRB, progress, safety, AE, and other reports
- Report at the participant, study, or multi-study-level, filter reporting as needed.
-
Publish Faster
-
Directly connect study data to manuscript ready tables, update on demand.
-
Centralize and organize manuscripts, data sets, files, etc.
-
Overcome REDCap Limitations
Unusable Data
The data can be unusable, incomplete, invalid, inaccessible, etc.
Delayed Changes
Changes can be technically involved, delayed, and risk data loss.
Nothing For Publishing
No tools for analyses and publication of results, or to organize academic output.
High Dropout Rate
Participants often don't complete data, lose interest, and/or dropout.
Poor Scaling Features
Unable to connect and manage research projects within a single environment.
Unhappy Users
There is a steep learning curve and the UI is difficult to navigate.
No Clinical Utility
Real-time clinic use of data impossible, and/or has no practical use.
No Patient Benefits
Only thing participants can do is fill out forms, often leading to dropouts.
Inflexible Features
Features don't match workflow and have limited setup options.
Can't Get Data
Extracting data is often cumbersome and limited to raw data.
Siloed Data
The data isn't accessible across studies requiring duplicate data entry
Poor Support
Difficult to get timely and accurate support and assistance when needed.
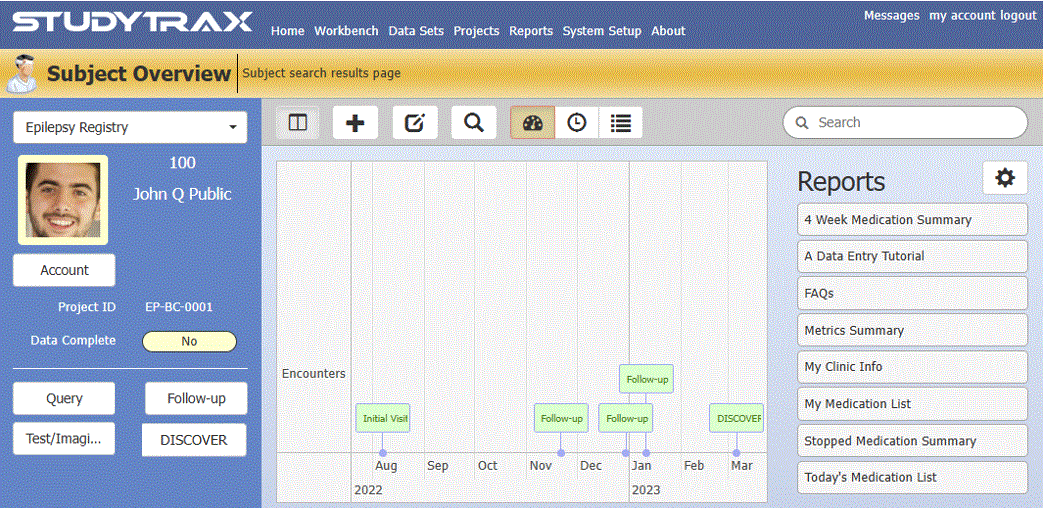
Work With A Clean, Intuitive UI
(Only See What's Relevant: Minimize Learning Curve)
REDCap UI
(Push Out Everything)

Participants Experience An Interactive Portal
(Auto-Resume Data Entry, Messaging, Incentive Points, Payment, Consent, Personalized Information)

Participants Experience Complicated Engagement
(Data Entry Only, Manual 'Return Code' Process)




